Chances and challenges
Enzymatically orchestrated cation polyene cyclization and rearrangement cascades in the biogenesis of terpenes and steroids are exceptional examples for nature’s ability to convert simple precursors into elaborated polycyclic frameworks. Learning from nature by mimicking these transformations can inspire efficient syntheses. In most cases, though, validated intermediates and enzymes are unknown, and biogenesis proposals are only speculations.
Development of robust and selective routes to access “drugable” natural products modulating the immune system, targeting cancer, and combating pathogens can so provide material for collaborative efforts.
Bringing together technology and synthesis is an important part of our work. We design and build novel reactors to enable and facilitate synthetic operations for environmentally and economically more sustainable approaches towards complex molecular entities and to handle highly reactive and short-lived intermediates.
Natural product synthesis
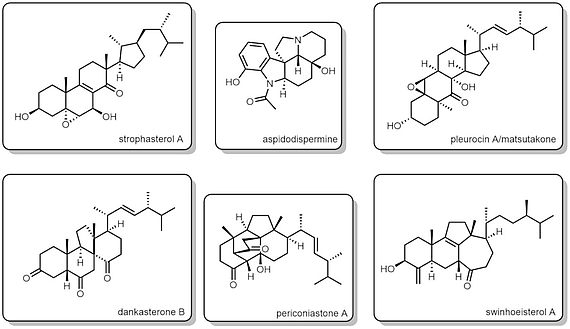
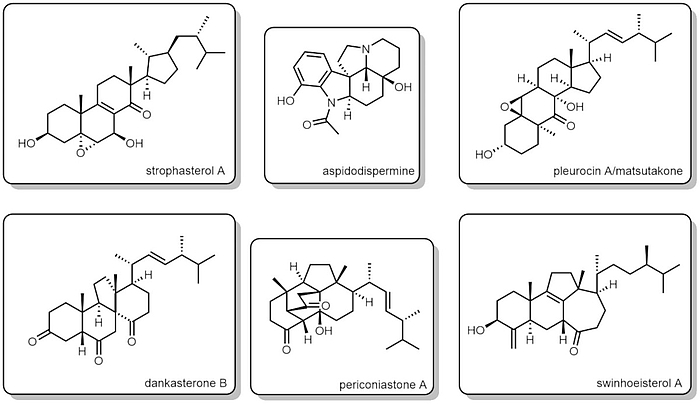
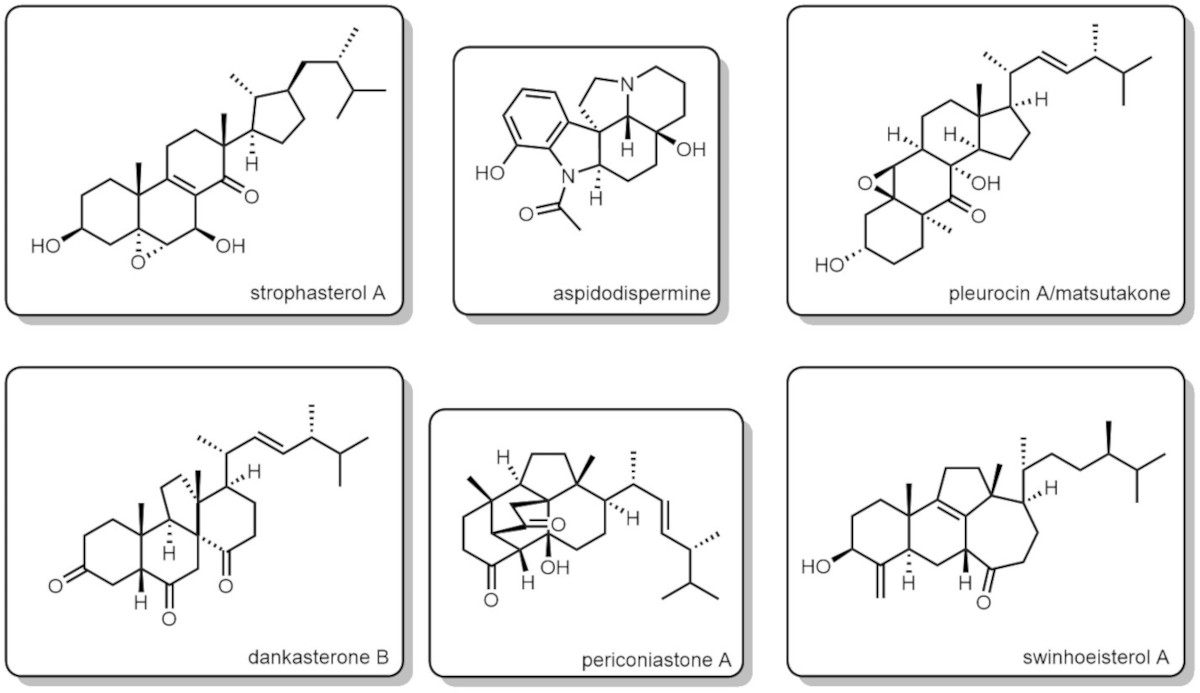
Currently, our synthetic targets are abeo-steroids, triterpenoids, and alkaloids. Find some natural products we have recently synthesized:
- strophasterol A
- aspidodispermine
- pleurocin A/matsutakone
- dankasterone B
- periconiastone A
- swinhoeisterol A
Flow technology
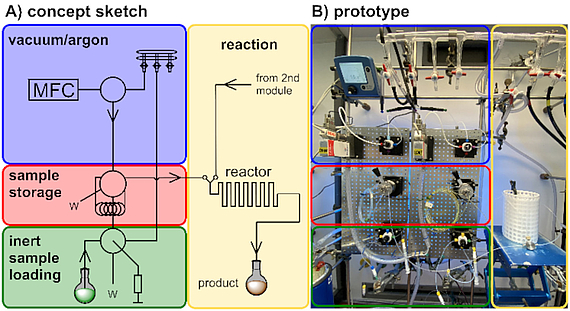
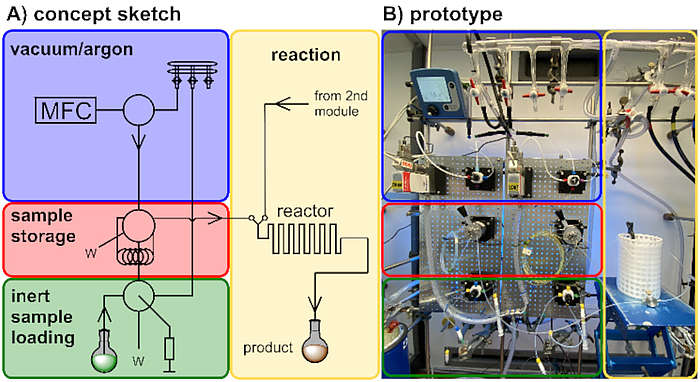
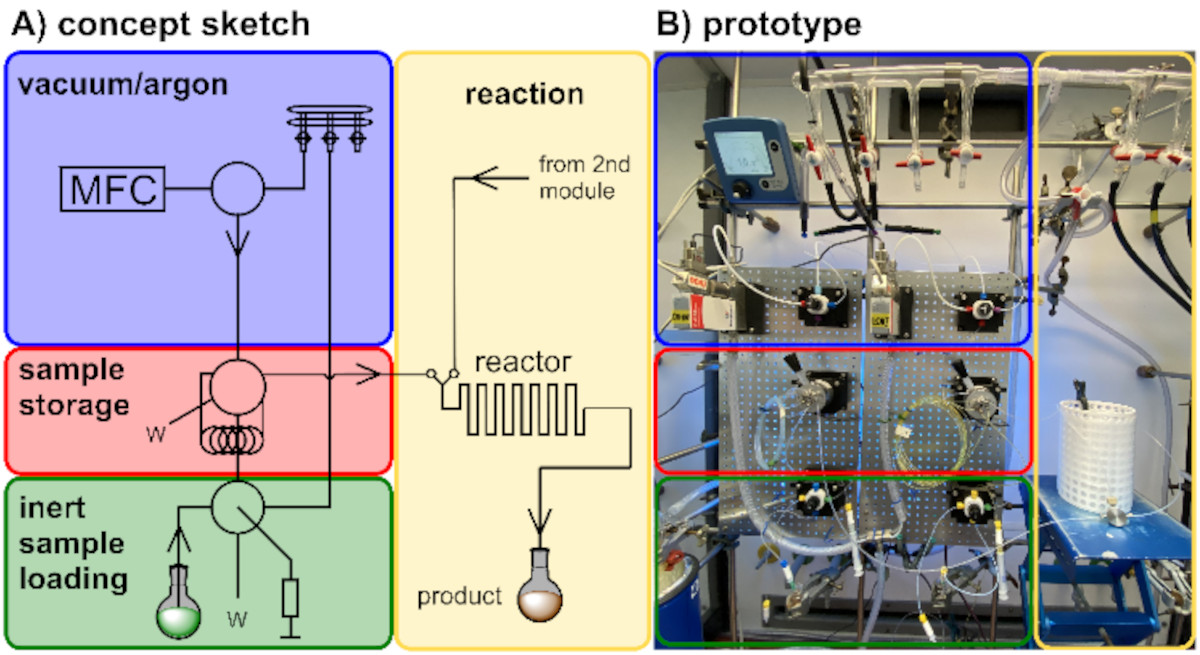
Our design and development programme focuses on novel continuous flow technology and its application in the synthesis of natural products and biologically relevant molecules. Development of new reactors, along with suitable reactions, and an understanding of their optimization potential is the basis for these endeavors.
Head of the group


30167 Hannover







